Researchers in the lab of Christopher C. Cummins are engaged in a variety of synthetic chemistry research projects. This research is broadly applicable to the fields of inorganic, organic, biological, and physical chemistry. We seek to synthetically access new and unusual compounds, including highly strained molecules or reactive intermediates. These compounds are then analyzed through a variety of spectroscopic and physical techniques ranging from X-ray crystallography to rotational spectroscopy. These measurements are then compared to the results of quantum chemical calculations to understand the fundamental properties and reactivity of these novel structures. Additionally, we target the synthetic utility of these compounds to address outstanding issues of synthetic or industrial importance, such as the efficient utilization of phosphate minerals. Through strong collaborations with fellow MIT researchers as well as researchers across the globe, we are able to undertake exciting chemistry applicable to a diverse range of fields and applications. While many projects focus on phosphorus containing compounds, the Cummins lab is broadly interested in main group and transition metal chemistry as well as crucial organic synthesis. We are fortunate to have this work supported by both the NSF and the NIH as well as a number of corporate and academic partners. Graduate students, post doctoral researchers, undergraduate researchers, and visiting students often pursue a new, exciting research direction. However, a few key, current research directions in the Cummins group follow.
Synthesis of Reactive and Strained Molecules
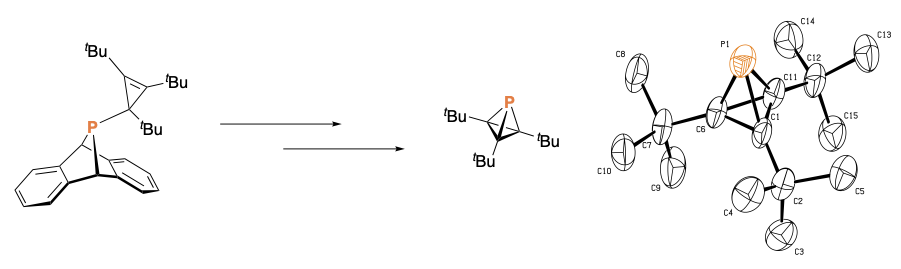
The Cummins group has always been interested in the generation and study of reactive small molecules as well as the trapping and reactivity of these compounds. Initial research often focused on transition metal complexes of these compounds, and recent research often focuses on the synthesis of suitable organic precursors. A powerful motif in this chemistry is embodied by RPA compounds (A = anthracene), sources of the RP phosphinidine moiety bound to an anthracene leaving group. Depending on the nature of the substituent, these compounds are able to release reactive phosphinidene species into the solution or gas phase either for further reactivity or spectroscopic characterization. Such platforms have been used to generate a wide variety of reactive small molecules including P2 and phosphaalkynes. We are currently exploring the effect of new R substituents such as aryl groups as we endeavor to generate new phosphorus containing compounds with applications as ligands and as possible sources of interesting reactive fragments. Reagents with incipient anthracene leaving groups are useful as group transfer reagents for generation of systems featuring reactive metal-ligand multiple bonds.
Recent Highlights
- With alkyl R substituents, RPA compounds are not readily cleaved under thermal conditions. Instead we have turned towards transition metal catalysis to generate strained three membered phosphoranes.1
- This RPA platform has allowed us to access unusual phosphinidene and phosphinidineoid compounds able to generate new phosphorus containing compounds. We have therefore synthesis a phosphatetrahedrane, a highly strained molecule reminiscent of both tetrahedrane and elemental P4.2
Sustainable Phosphate Reduction and Utilization
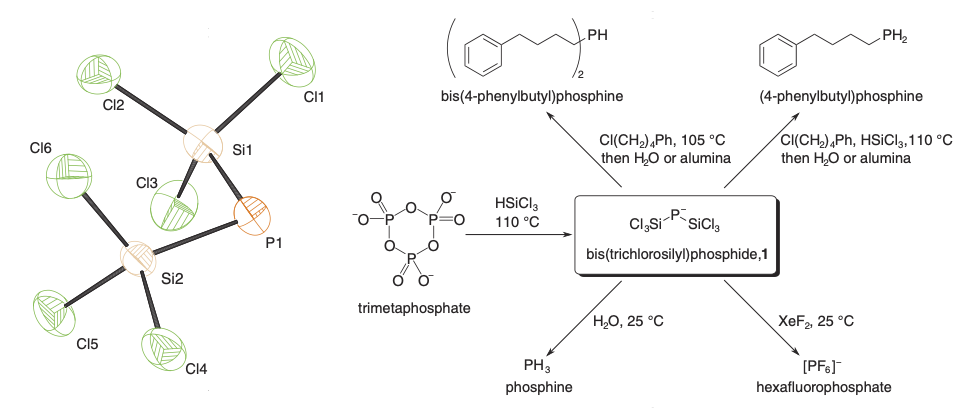
All phosphorus containing compounds are ultimately derived from phosphate rock minerals. The vast majority of this resource is cheaply converted to phosphoric acid, an essential fertilizer component. All reduced phosphorus and phosphorus-carbon bond containing compounds are instead produced from the energy intensive carbothermal reduction of phosphate to white phosphorus (P4). We seek to render obsolete this energy intensive process by converting phosphates directly to value added reduced phosphorus containing compounds, circumventing redox inefficient and dangerous white phosphorus.
Recent Highlights
- Treatment of various phosphate sources with trichlorosilane leads selectively to a new anion, bis(trichlorosilyl)phosphide anion, isolated as it tetrabutylammonium salt. This reduced and totally deoxygenated compound is able to form a variety of value added phosphorus containing chemicals for example through P-C bond forming reactions with suitable electrophiles or the reaction with xenon difluoride to give hexafluorophosphate anion.3
- The mechanism of trichlorosilane reduction of phosphate was investigated and found to produce hydrogen gas as a side product. Furthermore, trichlorosilane reacts with sulfate, generating trichlorosilyl sulfide, isolated as its tetrabutylammonium salt. This anion is furthermore capable of sulfur-element bond formation through treatment with suitable electrophiles.4
Selective Synthesis of Oligophosphates and Application to Biochemical Studies
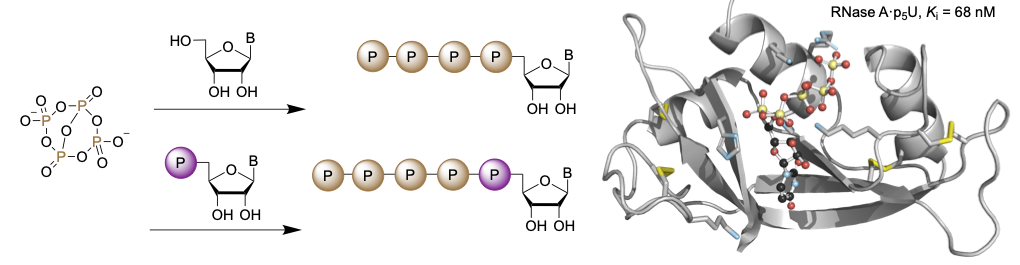
Our group has worked extensively on phosphorus oxyanions, especially metaphosphates, cyclic oligomers of the PO3− anion. Utilizing lipophilic cations such as bis(triphenylphosphine)iminium and tetrabutylammonium, we have isolated a variety of protonated or dehydrated phosphate compounds which would be inaccessible in aqueous solutions. We have furthermore utilized these compounds as ligands for transition metals to stabilize terminal oxo species or act as electrolytes for redox flow batteries. Recently, we have applied these dehydrated and activated phosphates as phosphorylation reagents. These new reagents are able to efficiently introduce long oligophosphate chains in one transformation. Furthermore, the resulting cyclic intermediates can be further functionalized and ring opened to access terminally disubstituted oligophosphates with varying phosphate chains lengths. Current research is aimed at the selective introduction of even longer chains a new complex morphologies. With the Raines lab (MIT), we are actively engaged in collaborative biochemical applications for these oligophosphates as inhibitors of Ribonuclease A.
Recent Highlights
- We reported an activated trimetaphosphate reagent that is capable of delivery triphosphorylating a wide variety of C, N, and O nucleophiles.5
- Utilizing a dehydrated tetrametaphosphate we previously reported, we selectively synthesized nucleoside tetra- and pentaphosphates. In collaboration with the Raines lab (MIT), we showed the utility of these compounds as inhibitors of Ribonuclease A.6
References
- Geeson, M. B.; Transue, W. J.; Cummins, C. C. J. Am. Chem. Soc. 2019, 141, 13336–13340. DOI: 10.1021/jacs.9b07069
- Riu, M.-L. Y.; Jones, R. L.; Transue, W. J.; Müller, P.; Cummins, C. C. Sci. Adv. 2020, 6. DOI: 10.1126/sciadv.aaz3168
- Geeson, M. B.; Cummins, C. C. Science 2018, 359, 1383–1385. DOI: 10.1126/science.aar6620
- Geeson, P., Michael B and Ríos; Transue, W. J.; Cummins, C. C. J. Am. Chem. Soc. 2019, 141, 6375–6384. DOI: 10.1021/jacs.9b01475
- Shepard, S. M.; Cummins, C. C. J. Am. Chem. Soc. 2019, 141, 1852–1856. DOI: 10.1021/jacs.8b12204
- Shepard, S. M.; Windsor, I. W.; Raines, R. T.; Cummins, C. C. J. Am. Chem. Soc. 2019, 141, 18400–18404. DOI: 10.1021/jacs.9b09760
Note: any opinions, findings, and conclusions or recommendations expressed in this web site are those of the author(s) and do not necessarily reflect the views of the National Science Foundation or the National Institutes of Health.
